Mass Percent of Water in Copper Sulfate
The compound is called copperII sulfate pentahydrate and if we wish to find the percent by mass of water we need to find the molar mass of the hydrate first. Mass of the test tube and unknown sample 14653g.

Friday March 6 Objective Determine The Ratio Of Water Molecules To Copper Sulfate Molecules In A Blue Chemical Checkpoint How Many Moles In 3 54 Grams Ppt Download
63546 32065 1599944 Percent composition by element.

. Percentage of Water Amount of water in Copper Sulfate Pentahydrate Mass of Copper Sulfate Pentahydrate used Percentage of water. H2O mass HO x 100 mass of hydrate The actual percentage of water in copperII sulfate pentahydrate is 360. 6355 g mol1 6355 3206 4 15999 g mol1 100.
Nov 24 2012 H2O mass H2Omass sample100. Assume one copper atom per formula unit or n1. CuSO4xH2O where x is the number of hydrates Assume 100 g sample 284 g of water 716 g of copperII sulfate.
Finally What is the mass percent of water in copper II sulfate heptahydrate It also means that copper sulfate pentahydrate contains 100 6392 3608 percent water by mass. When determining the formula mass for a hydrate the waters of hydration must be included. A mass of water contains 06 by weight.
What is the percent water in copperII sulfate pentahydrate CuSO 4 5 H 2 O. Commercial copper sulfate is usually about 98 pure copper sulfate and may contain traces of water. The hydrate is CopperII Sulfate or CuSO4 After conducting the experiment I found the mass percentage of this compound is.
Chemistry questions and answers. A 350 alcohol content makes ushydrous copper sulfate. See answer 1 Best Answer Copy there are 1596g of cupric sulfate and 901g of waters635gCu321gS 1604gO1596gCuSO4 5 1012gH5 160gO901g5H20 therefore the gram formula mass is.
Its mass is 19 percent sulfate by volume and its hydrous form is 25 percent. Commercial copper sulfate is usually about 98 pure copper sulfate and may contain traces of water. By mass CuSO4 100 mCuSO4msample.
Commercial copper sulfate is usually about 98 pure copper sulfate and may contain traces of water. There were many factors that may have affected the results of the experiment. A 13 percent sulfate content is found in 47 of the water.
16 g 34 Conclusion The percentage of water in Copper Sulfate was found to be 34. Convert grams CopperII Sulfate to moles or moles CopperII Sulfate to grams. The percent by mass of water of hydration is calculated as the mass of water of hydration divided by the mass of hydrated copper sulfate.
CopperII Sulfate molecular weight. 1 Cu6355 gmol 1 S3207 gmol 4 O1600 gmol 15962 gmol Formula mass 15962 gmol 5 H 2 0 1802 g H 2 0mol 24972 gmol 2. Calculate the percent by weight of copper in copper II sulfate CuSO 4.
Molar mass of copper Molar mass of copper sulfate 100 Explanation. Also Know what is the mass percentage of water in copper sulfate. The equation you need for this calculation is in the following lab write up.
Anhydrous Copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. It also means that copper sulfate pentahydrate contains 100 - 6392 3608.
Anhydrous Copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. Cu 6355 gmol. S 3207 gmol.
The theoretical actual percent hydration percent water can be calculated from the formula of the hydrate by dividing the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiplying by 100. Enter your values and then click Calculations. Molar mass of CuSO4 1596086 gmol.
Note that the anhydrous salt is white but it dissolves in water to give a beautiful blue solution. Calculate the error for each trial. The correct percentage of water is 36.
In the formula CuSO 4 x H 2 O x is calculated as the amount of water of hydration divided by the amount of anhydrous copper sulfate. 71 percent copper 60 percent zinc. 284 water 716 copperII sulfate This is how I find the number of hydrates in the solution.
Sulfur content 82 and carbomer content 373. Chemistry Determine the percent by mass of water in your sample of hydrated copper II sulfate. It contains about 19 percent sulfate by mass and 25 percent sodium in its blue-soluble form.
Copper makes up 47 percent and soybeans make up 38 percent. If you calculate the mass percent of water in your copper sulfate pentahydrate compound using your experimental data and find thaf it is too high what do you rhink happenex. A copper content of 47 as opposed to 38.
In extension the percentage of water in the hydrated copper II sulfate compound was 3215. The amount of sulfate in 47 of the solution is 1244. Experimental value - actual value error actual value X 100.
CuSO4 5H20 Round to three sig figs 1 See answer. DrBob222 Nov 24 2012 Respond to this Question Similar Questions. Calculate the molar mass of an unknown copper compound from the data below.
Ferrous sulfate heptahydrate What is FeSO4 7H2O. Copper has an 81 percent content and aluminum has a 60 percent content. What is the formula for iron II sulfate heptahydrate.
Once you know the mass of copper sulphate you can calculate the mass percentage as follows. Mass of water 095 g Mass of anhydrous copper sulfate 219 g Mass of hydrate copper sulfate 314 g ℹ A. Up to 24 cash back The calculated mass of water lost from the hydrated copper II sulfate compound was 0451.
Calculate the formula mass. This results in the hydrate formula of CuSO4 5H20.
Tribasic Copper Sulfate Cu3h2o10s2 Pubchem
What Is The Percentage Of Copper Sulphur And Oxygen Elements In Copper Sulphate Cuso4 Quora
Copper Sulfate Pentahydrate Cuso4 5h2o Pubchem

Copper Sulfate Crystals Experiment Mel Chemistry

Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram

Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram

Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram

Purification Of Copper Sulfate 5 Steps Instructables

Copper Sulfate Pentahydrate 99 9 Sciencekitstore Com

Copper Sulfate Pentahydrate 100 Blue Vitrol 2 Lbs Sciencekitstore Com

Determination Of The Amount Of Water Present In Copper Sulfate Hydrate International Baccalaureate Chemistry Marked By Teachers Com
Why Is Copper Sulfate Blue And What Is It Used For Quora
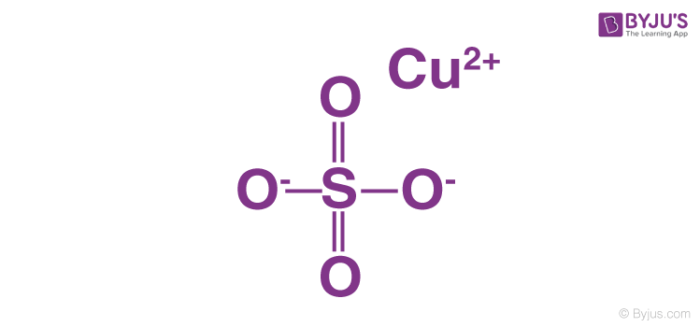
Copper Sulfate Structure Properties And Uses Of Cuso4

Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram

Percentage Composition Of H2o In Cuso4 5h2o Youtube

Best Ammonium Copper Ii Sulfate Hexahydrate Nh4 2cu So4 2 6h2o Crystalline Copper Pure Products Chemical Formula

Question Video Writing The Equation For The Reaction At The Anode During The Electrolysis Of Copper Sulfate Solution Using Inert Electrodes Nagwa

Comments
Post a Comment